A test to detect the potentially deadly pregnancy complication preeclampsia. A brain-to-device connection that produces natural movement for amputation patients. Bioengineered skin in the shape of a full human hand to help burn victims heal.
Below we describe these and other medical advances that aim to improve the lives of millions of people.
Hearing for the first time

For 11 years, Aissam Dam heard nothing. Today he can hear birds chirping, friends laughing, and the sound of his father’s voice.
Aissam is the first patient in the United States to undergo an experimental gene therapy for a rare form of congenital deafness.
Dam’s physicians at Children’s Hospital of Philadelphia (CHOP), who operated on him in October 2023, aimed to replace the boy’s otoferlin (OTOF) gene. Normally, hair cells in the inner ear use an OTOF protein to help ferry sound signals to the brain. When the gene doesn’t work, the connection fails, explains CHOP otolaryngologist John Germiller, MD, PhD.
Germiller’s team used a minimally invasive surgical approach and a custom-made device to create a tiny opening through a membrane called the round window. They then inserted a viral vector — a virus that doesn't cause disease — to carry the gene to the hair cells in the inner ear. Over the next several weeks, the hair cells gradually began producing the missing protein, notes Germiller, a principal investigator studying the gene therapy, which was developed by the biotechnology firm Akouos Inc.
“It has gone astoundingly well. Aissam went from profound deafness to mild-to-moderate hearing loss, in which sound is a little muffled,” says Germiller. “The field has been working toward this goal for decades. It was a dream come true.”
CHOP is one of several sites around the world conducting trials using gene therapy to address OTOF-related deafness. Though the trials differ slightly, all ultimately aim to offer another option besides cochlear implants, which require more invasive surgery and restore less hearing, Germiller explains. “While cochlear implants work remarkably well in some ways, they have only up to 22 channels, while natural hearing involves over 3,000 channels.”
Now that the first stage went smoothly, CHOP researchers are able to enroll more trial patients, including infants. And looking ahead, they hope to see additional gene therapies developed to address hearing loss from other genes.
Meanwhile, Germiller reports that Aissam is thrilled by the many sounds he can hear. “He says, ‘I like it all. I like it all the time.’”
A breakthrough bionic limb
People with prosthetic legs often have trouble navigating bumpy terrain, climbing stairs, and generally walking with a natural gait. That’s because bionic limbs move using preprogrammed computer algorithms.
But what if a prosthetic limb could instead respond to the user’s brain?
That was the question raised by a team of researchers at Brigham and Women’s Hospital (BWH) and the Massachusetts Institute of Technology in Boston. The result, published in Nature Medicine this month, is a surgical process and a robotic device that produce dramatically better movements.
“Since the time of the Civil War, the standard amputation technique has remained largely unchanged,” says Matthew Carty, MD, a BWH reconstructive plastic surgeon and a study author. “In the new approach, we treat the residual limb much differently.”
In that process — known as agonist-antagonist myoneural interface (AMI) construction — Carty reconnects thigh muscles that are usually severed.
That reconnection allows the brain to send signals to contract the muscle, and the contractions produce electrical signals that tell a prosthetic leg how to move. This helps even when patients use a traditional prosthesis. But when using the novel prosthesis, tiny computers in it can send signals back to the muscles that then shoot crucial information on to the brain.
“Since the time of the Civil War, the standard amputation technique has remained largely unchanged. In the new approach … it’s as if the brain has been fooled into thinking the limb is still there.”
Matthew Carty, MD
Brigham and Women’s Hospital
Patients who received the AMI procedure — called the Ewing amputation, after its first recipient — and the novel prosthesis were able to walk at about the same rate as people without amputations. They could also stay stable on uneven terrain, navigate obstacles, and climb stairs more easily than did a comparison group of patients.
“It’s as if the brain has been fooled into thinking the limb is still there,” Carty says. That’s a tremendous benefit, he notes, because prosthesis recipients often feel an unpleasant disconnection from the devices they wear.
The amputation procedure is already available at BWH, and Carty hopes the novel prosthesis will receive Food and Drug Administration (FDA) approval in the next five years or so. Such an advance could offer hope to many of the nearly 2 million Americans who live with limb loss, a number that’s expected to double in the next few decades, mostly because of rising rates of diabetes.
Carty is excited about the possibility of better care for amputation patients. “It’s a gift and a privilege to feel like you can impact the lives of fellow humans. This is why we go into medicine.”
Better help for burn victims
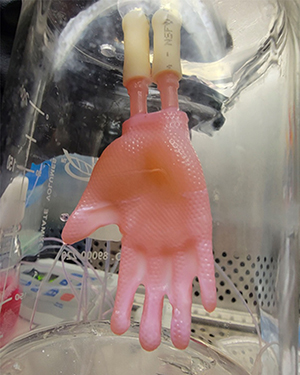
When they treat a burn victim, physicians often sew on artificial skin to protect the wound and promote healing. But those bits of skin come in small patches that require lots of suturing and often don’t conform well to complex human shapes.
Now researchers have created an entire human hand of bioengineered skin that fits as neatly as a glove.
“Usually we need to use many patches, maybe even 15 per hand, and have to carefully wrap them around each finger. That makes the operations take much longer,” says Hasan Erbil Abaci, PhD, a bioengineer in the dermatology department at Columbia University Vagelos College of Physicians and Surgeons in Manhattan. It can also lead to more scarring, pain, and other complications.
The new method, described in Science Advances, is designed to allow physicians to simply slip on a custom-made, continuous sheath and place a few stitches or bandages at the wrist.
The glove — the first major redesign of engineered skin grafts in 40 years — is stronger than the pieced-together patches and ideally will produce more aesthetic, better-functioning results for patients, says Abaci, who led the effort.
Although the 3D skin glove can’t be used for human patients yet, even creating it was an achievement. First, the researchers performed a laser scan of a person’s hand. Next, they crafted a scaffold using computer-aided illustrations and 3D printing. The structure was then seeded with the cells necessary to generate human skin and permeated with a medium similar to blood to nourish the growing cells.
Before the hand can be tried on humans, the approach must first be tested extensively on animals. So far, it’s done well. “We used [a form of the glove] on the hind legs of mice, an area that’s very hard to graft. It went very smoothly and quickly, like putting on a pair of shorts tailored in the mouse’s personal size,” says Abaci.
Abaci acknowledges that animals differ from people, but he’s confident that the approach will succeed on humans. “We already know that bioengineered skin works well in humans. So this approach isn’t different in biology, just in geometry.”
In fact, the 3D skin has worked better than Abaci expected. “We thought we were just avoiding the problems of the patches, but the cells surprised us,” he says. “They seemed to sense the shape and to change their structural properties in response. It was fascinating to see how well they responded to the shape we provided them.”
Stroke patients regain arm movement

Cutting food, gripping a pen, getting dressed — all those simple tasks can be impossible for someone who has suffered a stroke.
But for one amazing month, thanks to researchers at the University of Pittsburgh and Carnegie Mellon University, participants in a study on spinal cord stimulation regained abilities they had lost long ago.
The study, published in the journal Nature Medicine, is the first successful attempt to use such stimulation to treat paralysis and weakness in the arms and hands of stroke patients.
“The effect was dramatic,” says George Wittenberg, MD, PhD, medical director of the Rehab Neural Engineering Labs and a neurology professor at the University of Pittsburgh School of Medicine. “Without simulation, one patient couldn’t get a fork to her mouth [with her left hand]. With it on, we watched her eat one of her favorite foods, chicken nuggets.”
In fact, with stimulation — which was tailored for each patient — participants quickly experienced greater strength, function, and range of movement, Wittenberg says.
To achieve these effects, researchers implanted two lines of eight electrodes alongside the spinal cord. The approach took advantage of the fact that even after a stroke, the brain tries to send signals through the spine to nerve cells. The stimulation makes the nerves more receptive to those signals, Wittenberg adds.
The experimental process may offer hope to millions of patients. More than 12 million people worldwide have a stroke each year, and nearly three-quarters of them experience long-term movement loss in their arms and hands.
The stimulation trial was more successful than researchers anticipated. The study protocol required removing the electrodes after 29 days, but even a month after removal, patients still retained some improved abilities.
“It feels like we are possibly entering a new era in making meaningful differences in stroke patients’ lives,” Wittenberg says.
Next steps toward approval by the FDA include a trial comparing electrode stimulation plus occupational therapy with occupational therapy alone. Meanwhile, patients who benefited from the approach hope it will one day be available to them again.
“I did threaten to not show up to the surgery to get it removed,” quipped one participant, Heather Rendulic. “I just wanted it all the time.”
Predicting dangerous preeclampsia
Patients who develop preeclampsia during pregnancy can suffer dangerously high blood pressure, organ damage, and even death. The fetus can face serious risks as well, including preterm birth and stillbirth.
Preeclampsia affects 1 in 25 pregnancies in the United States, and it is one of the leading causes of maternal mortality. It’s also responsible for more than 500,000 fetal deaths worldwide each year.
For decades, physicians had only rudimentary methods — blood pressure readings, for instance — for assessing whether a patient might be at risk of developing severe preeclampsia. But now a simple blood test can help identify with high accuracy which patients face such a risk.
“Our test outperforms all others, both in predicting who will get severe preeclampsia and who won’t,” says Sarosh Rana, MD, MPH, the test’s lead investigator and chief of maternal-fetal medicine at the University of Chicago Medicine. “For example, it’s 96% accurate in ruling out severe preeclampsia.”
The test, which received approval from the FDA in May 2023, works by measuring a ratio between two blood proteins, sFlt1 and PlGF. It can be used between 23 and 35 weeks of pregnancy and is meant for patients with hypertension or symptoms of it. (It is not meant for patients carrying more than one fetus, though.)
“Our test outperforms all others, both in predicting who will get severe preeclampsia and who won’t.”
Sarosh Rana, MD, MPH
University of Chicago Medicine
It’s crucial to identify pregnant patients who require additional monitoring or hospitalization, Rana says. But she adds that it’s also important to determine who does not need extra tests or treatment. “There is no cure for preeclampsia other than preterm delivery. We don’t want to deliver babies prematurely unless absolutely necessary.”
Rana notes the test’s potential impact on health inequities too. “Black pregnant people are 60% more likely than white people to develop preeclampsia,” she says. “They also have much higher rates of complications and death from it.”
The study, which enrolled more than 1,000 participants at 18 sites, measured patients’ blood proteins and then watched to see if those whose blood ratio suggested a risk of severe preeclampsia were in fact more likely to develop it. The results, which led to the FDA approval, were published in NEJM Evidence in 2022.
Rana also developed a protocol to guide providers in making such key decisions as when to use the test and how best to manage and counsel patients based on its results.
Looking ahead, Rana hopes for even more progress. “The best thing in my life would be a treatment to cure preeclampsia. We’ve made a good start to understanding the mechanisms involved. It would be great if we could go even further to help our moms and babies.”

